Absorption
When a fiber absorbs, less power will be seen at the output than was put into the fiber. Absorption is any mechanism by which the material takes energy into its structure. This commonly happens as electrons in the glass move to excited states, sometimes escaping if a lot of energy is incident. Light can be considered as discrete packets of light energy, or photons with the energy each carries dependent upon the wavelength of the light.
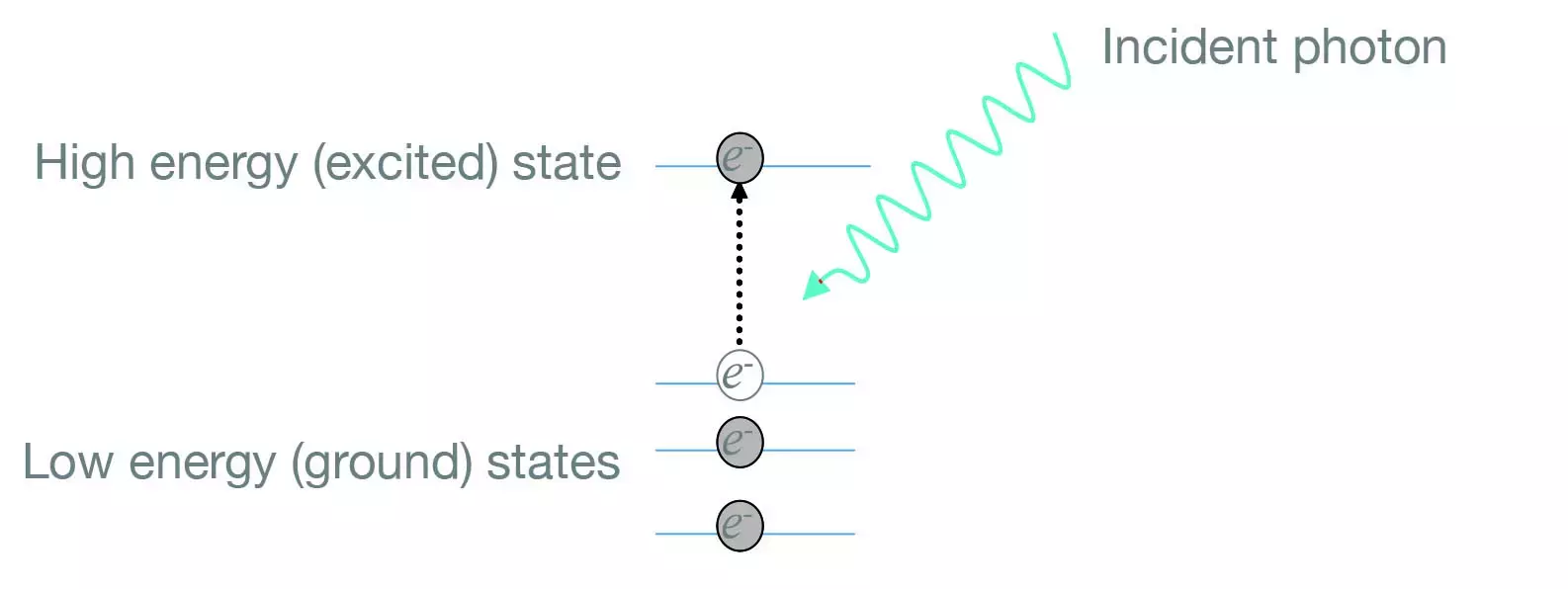
Sometimes the lifetime of the excited state is very short and light is almost instantaneously re-emitted, though not necessarily in the incident direction, and the absorption is seen as a scattering mechanism.
The strongest absorption occurs when the energy of the photons in the light, match the energy difference amongst the energy states that the electron jumps between. Electronic absorption in atoms of rare-earth metals elements is a fundamental part of the operation of amplifier, laser and ASE fiber sources. The main excited states of rare-earth elements have relatively long lifetimes, and so when pumping at a wavelength that matches the energy gap between an excited and ground state, lots of the atoms can be excited together (termed Population Inversion) ready to give up their energy via either spontaneous emission or stimulated emission.
In addition, absorption at longer IR wavelengths causes the molecular bonds across the glass lattice to vibrate, warming the glass. Absorption by these bonds, typically at UV wavelengths, can also be associated with defects in the lattice shifting or re-ordering, restructuring the lattice. This can be a particularly useful mechanism in some applications, as maximizing it leads to strongly photosensitive fibers.
The term is invariably used when discussing loss associated with a core dopant that has specific absorption bands, which are fundamental to the element. These occur at very specific wavelengths and coincide with fundamental energy states within the ion, which form the core dopant. These are usually confined to rare-earth ions within the product portfolio of Fibercore. The absorption bands are so strong that the absorption is quoted per meter and not per kilometer. For example Fibercore produce ytterbium doped fiber that has peak absorption of 1000dB per meter. Absorption is measured in much the same way as attenuation, but the lengths are selected appropriately for the amount of absorption in the fiber.
Absorption spectra
Each atom, ion and molecule has a characteristic wavelength band of over which it will absorb, and the strength of absorption varies in a unique way with wavelength for each. The precise shape of the spectrum absorption of rare-earth ions also depend upon the exact composition of the host glass, being modified by the presence of co-dopants.
Absorption cross section,σabs
The probability that there is an ion in a given cross sectional area that will absorb an incident photon; units are area [cm2].
Absorption coefficient, α(λ)
How strongly a material will absorb. It is quantified by the product of the absorption cross section and doping density (N0). This is a Giles parameter used erbium doped fiber amplifier models.
Related Products: Dual Clad Erbium/Ytterbium Doped Fiber, Erbium Doped Fiber IsoGain™, Erbium Doped Fiber MetroGain™, GainMaster™ Simulation Tool, Highly Germanium Doped Fiber, PM Erbium Doped Fiber, SM Erbium/Ytterbium Doped Fiber, SM Nd Doped Fiber, SM Ytterbium Doped Fiber
Related Terms: Active Medium, Aluminosilicate Glass, Amplified Spontaneous Emission, Amplifier, Clustering, Cross section, Emission Measurement, Emission Spectra, Erbium Doped Fiber (EDF), Erbium Doped Fiber Amplifier (EDFA), Erbium, Excited State, Excited State Absorption (ESA), Gain Coefficient, Giles Parameter, Ground State, Laser, Non-Radiative Decay, Stimulated Emission

