Defects
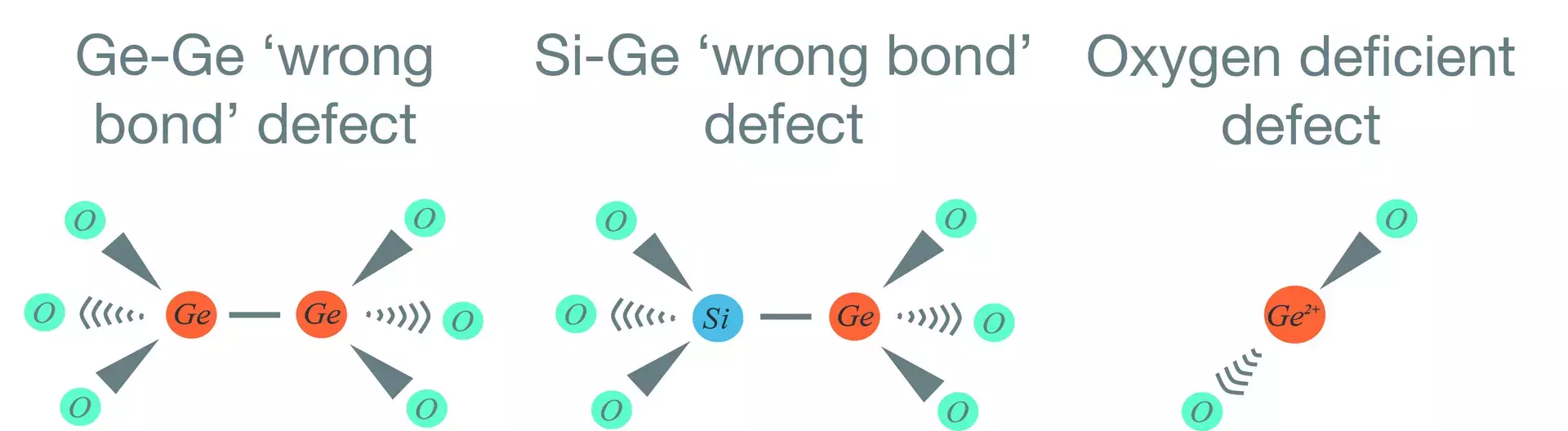
In a perfect germanosilicate glass, the silicon atoms and germanium doping are evenly distributed throughout the glass, and there is plenty of oxygen in the structure linking the semiconductor atoms with O-Si-O or O-Ge-O bonds. Wrong bonds, or defects, are where semiconductor elements form direct links with Si-Si, Ge-Ge or Ge-Si bonds. Deficiency centers are regions in the glass lattice where there are not enough oxygen atoms available to form the required bonds, and the semiconductor atoms are left with incomplete links into the lattice. In this case the semiconductor atoms may have spare valence electrons and form ions, for example Ge2+, at these points.
Related Terms: Wrong Bond